Navigating Global Pharmacopeia Standards and Water Quality for Critical Utilities

Adherence to stringent pharmacopeia standards is paramount for ensuring product quality, safety, and regulatory compliance in pharmaceutical manufacturing. As leaders in drug development and production set the pace for the industry, the significance of water quality for critical utilities in meeting these standards cannot be overstated.
Understanding Pharmacopeia Standards: A Foundation for Quality Assurance
Pharmacopeias, established by regional or national authorities, serve as comprehensive repositories of standards and quality specifications for medicines within a specific jurisdiction. These standards encompass crucial parameters such as directions, dosages, and quality control measures, forming the regulatory framework that governs pharmaceutical production.
Pharmacopeia Specifications for the United States, Europe, and Asia
A pharmacopeia is a legally binding compilation of standards and quality specifications prepared by a regional or national authority for the medicines used in a specific region or country. These specifications are critical for directions, dosages, and other vital factors essential for ensuring the regulatory control of pharmaceuticals. The primary goal of international pharmacopeias is to establish a worldwide standard of uniform quality and usage specifications
USP, EP, and JP Pharmacopeia Standards
Countries develop national pharmacopeias to support the general health of their populations through consistent standards and specifications of medicines. Three of the most recognized organizations globally are the United States Pharmacopeia (USP), the European Pharmacopeia (EP), and the Japanese Pharmacopeia (JP).
United States Pharmacopeia (USP): As a leading independent, nonprofit organization, the USP sets the gold standard for quality, strength, and purity of medicines globally. Pharmaceutical companies worldwide rely on USP monographs to ensure compliance with stringent quality assurance parameters enforced by regulatory bodies such as the United States Food and Drug Administration (USFDA).
European Pharmacopeia (EP): The EP provides a comprehensive collection of legally binding monographs that outline standards for ingredients, dosage forms, and testing methods for the European pharmaceutical industry. Compliance with EP specifications is essential for accessing the European market and ensuring product acceptance.
Japanese Pharmacopeia (JP): The JP, which dates back to 1886, sets forth criteria, specifications, and standard test methods to guarantee the quality and safety of medicines in Japan. Pharmaceutical manufacturers worldwide collaborate closely with the JP secretariat to ensure alignment with the latest science, medicine, and technology advancements.
Chinese Pharmacopeia (CP): Emerging as a significant player in the global pharmaceutical landscape, the CP sets standards and specifications for medicines in China. With China’s growing influence in the pharmaceutical industry, adherence to CP guidelines is becoming increasingly important for companies seeking to access the Chinese market and ensure product quality.
Pharmacopeia Guidelines for Pure Water
Pharmaceutical companies use active pharmaceutical ingredients (APIs) to manufacture medicine. These ingredients are the elements that produce their intended effect, whether preventing, treating, curing, or diagnosing a specific condition or disease. Combination medicines contain more than one active ingredient that could act differently to treat various symptoms.
Pharmacopeia guidelines are critical for ensuring that all APIs act the same among different brand names, including generic drugs. Companies that use APIs to manufacture products must employ rigorous processes to promote high quality and compliance. One of the most vital parts of these processes is using the correct type of purified water for a specific manufacturing stage.
At MECO, we supply pharmaceutical companies with MASTERpak™ systems that deliver an efficient, easy-to-use purification solution. We design these systems with advanced technology to ensure the production of high-quality water that meets the necessary pharmacopeia guidelines.
Water Quality for Critical Utilities:
Ensuring Compliance with Pharmacopeia Standards
In pharmaceutical manufacturing, water is a critical ingredient for various processes, including formulation, cleaning, and quality control. The quality of water used in these critical utilities, including Water for Injection (WFI), Purified Water, and Ultrapure Water, directly impacts product quality and compliance with pharmacopeia standards.
 Water for Injection (WFI)
Water for Injection (WFI)
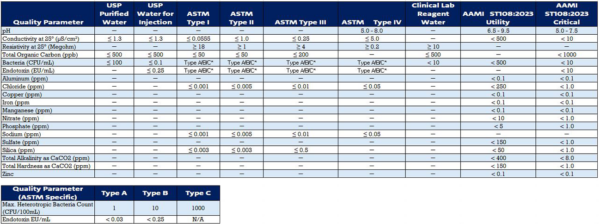
Water for Injection is the highest-quality water used in pharmaceutical manufacturing, meeting stringent pharmacopeia standards for purity and sterility. It is essential for reconstituting drugs, diluting formulations, and other critical processes where water purity is paramount.
United States Pharmacopeia (USP):
- Conductivity:
- Not more than 1.3 µS/cm at 25°C
- Total
- Organic Carbon (TOC): Not more than 500 ppb
- Bacterial
- Endotoxins: Not more than 0.25 EU/mL
- Microbial (Non-Compendial)
- Enumeration Tests: Not more than 10 CFU/100 mL
European Pharmacopeia (EP):
- Conductivity:
- Not more than 1.3 µS/cm at 20°C
- Total
- Organic Carbon (TOC): Not more than 500 ppb
- Bacterial
- Endotoxins: Not more than 0.25 EU/mL
- Microbial (Non-Compendial)
- Enumeration Tests: Not more than 10 CFU/100 mL
Japanese Pharmacopeia (JP):
- Conductivity:
- Not more than 2.1 µS/cm
- Total
- Organic Carbon (TOC): Not more than 500 ppb
- Bacterial
- Endotoxins: Not more than 0.25 EU/mL
- Microbial
- Enumeration Tests: Not more than 10 CFU/100 mL
Chinese Pharmacopeia (CP):
- Conductivity:
- Not more than 1.3 µS/cm
- Total
- Organic Carbon (TOC): Not more than 500 ppb
- Bacterial
- Endotoxins: Not more than 0.25 EU/mL
- Microbial
- Enumeration Tests: Not more than 10 CFU/100 mL
These standards ensure that Water for Injection (WFI) meets the required purity and quality criteria for use in pharmaceutical manufacturing processes. Pharmaceutical companies must adhere to these standards to maintain product integrity and comply with regulatory requirements in their respective regions.
Purified Water (PW)
Purified Water meets pharmacopeia specifications for chemical and microbiological purity and is used in various pharmaceutical processes, including formulation, cleaning, and laboratory testing. It is crucial for maintaining product integrity and ensuring compliance with regulatory requirements.
United States Pharmacopeia (USP):
- Conductivity:
- Not more than 1.3 µS/cm at 25°C
- Total
- Organic Carbon (TOC): Not more than 500 ppb
- Bacterial
- Endotoxins: Not more than 0.25 EU/mL
- Microbial
- Enumeration Tests: Not more than 100 CFU/mL
European Pharmacopeia (EP):
- Conductivity:
- Not more than 5.1 µS/cm at 20°C
- Total
- Organic Carbon (TOC): Not more than 500 ppb
- Bacterial
- Endotoxins: Not more than 0.25 EU/mL
- Microbial
- Enumeration Tests: Not more than 100 CFU/mL
Japanese Pharmacopeia (JP):
- Conductivity:
- Not more than 4.3 µS/cm at 25°C
- Total
- Organic Carbon (TOC): Not more than 500 ppb
- Bacterial
- Endotoxins: Not more than 0.25 EU/mL
- Microbial
- Enumeration Tests: Not more than 100 CFU/mL
Chinese Pharmacopeia (CP):
- Conductivity:
- Not more than 5.1 µS/cm at 25°C
- Total
- Organic Carbon (TOC): Not more than 500 ppb
- Bacterial
- Endotoxins: Not more than 0.25 EU/mL
- Microbial
- Enumeration Tests: Not more than 100 CFU/mL
These standards ensure that Purified Water meets the required purity and quality criteria for pharmaceutical manufacturing processes. It’s essential for pharmaceutical companies to adhere to these standards to maintain product integrity and comply with regulatory requirements in their respective regions.
Ultrapure Water (UPW)
Ultrapure Water surpasses pharmacopeia standards for water purity and is used in sensitive pharmaceutical applications, such as chromatography, molecular biology, and analytical testing. Its ultra-high purity level ensures accuracy and reliability in critical laboratory procedures.
United States Pharmacopeia (USP):
- Conductivity:
- Not more than 1.3 µS/cm at 25°C
- Total
- Organic Carbon (TOC): Not more than 500 ppb
- Bacterial
- Endotoxins: Not more than 0.25 EU/mL
- Microbial
- Enumeration Tests: Not more than 100 CFU/mL
European Pharmacopeia (EP):
- Conductivity:
- Not more than 0.1 µS/cm at 25°C
- Total
- Organic Carbon (TOC): Not more than 500 ppb
- Bacterial
- Endotoxins: Not more than 0.25 EU/mL
- Microbial
- Enumeration Tests: Not more than 10 CFU/mL
Japanese Pharmacopeia (JP):
- Conductivity:
- Not more than 0.1 µS/cm at 25°C
- Total
- Organic Carbon (TOC): Not more than 500 ppb
- Bacterial
- Endotoxins: Not more than 0.25 EU/mL
- Microbial
- Enumeration Tests: Not more than 100 CFU/mL
Chinese Pharmacopeia (CP):
- Conductivity:
- Not more than 0.1 µS/cm at 25°C
- Total
- Organic Carbon (TOC): Not more than 500 ppb
- Bacterial
- Endotoxins: Not more than 0.25 EU/mL
- Microbial
- Enumeration Tests: Not more than 100 CFU/mL
These standards ensure that Ultrapure Water meets the stringent quality requirements specified by pharmacopeial standards, making it suitable for use in sensitive pharmaceutical applications, analytical testing, and research. Pharmaceutical companies must adhere to these standards to maintain product integrity and comply with regulatory requirements in their respective regions.
Partnering with MECO for Comprehensive Water Purification Solutions
At MECO, our comprehensive approach to water purification solutions is tailored specifically for biotechnology, pharmaceutical, life sciences companies, and research and development. We engineer and manufacture all the necessary technologies to produce Water for Injection (WFI), Purified Water, Ultrapure Water, and Pure Steam, ensuring our clients have access to a complete range of industry-leading water purification solutions.
Driving Innovation and Excellence
With nearly a century of expertise in the water industry, MECO has established itself as a premier supplier of water purification solutions. Since 1928, we have been providing state-of-the-art systems tailored to meet the stringent requirements of pharmacopeia guidelines.
MECO takes pride in our equipment manufacturing facilities in Mandeville, LA, and San Diego, CA (Water Works), where we engineer and manufacture all the necessary technologies to produce Water for Injection (WFI), Purified Water (PW), Ultrapure Water (UPW) and Pure Steam. Backed by a world-class service team, MECO delivers unmatched quality, innovation, and reliability to our customers. Our commitment to innovation, quality, and sustainability drives us to continually push the boundaries of water purification technology. By partnering with MECO, pharmaceutical manufacturers can confidently achieve operational efficiency and product excellence, knowing they have the support of a trusted industry leader with unparalleled expertise and capabilities.
Connect with MECO Today
Discover how MECO’s industry-leading water purification solutions can seamlessly integrate into your manufacturing processes, ensuring compliance with international pharmacopeia standards and driving sustainable growth. Contact MECO today.